Wheat Pathology Report for 2024
Disease Evaluations of 2024 OSU Wheat Breeding Lines
We determined the reactions of 950 Oklahoma State University wheat breeding lines to fungal diseases leaf rust, stripe rust, powdery mildew and tan spot and to viral diseases barley yellow dwarf (BYD) and wheat soil-borne mosaic/wheat spindle streak mosaic (WSBM/WSSM) (Table 1). Evaluations against leaf rust, powdery mildew and tan spot were conducted in the greenhouse using artificial inoculations. Evaluations against stripe rust, BYD and WSBM/WSSM were performed in the field under natural infections. In 2024, the wheat research team generated a total of 21,681 disease data points for the OSU breeding lines. Disease testing in the greenhouse and field will continue to enable line advancement with disease resistance in the OSU wheat breeding program.
| Disease | Test Condition | Number of breeding lines - Seedling Stage | Number of breeding ines - Adult-plant Stage |
|---|---|---|---|
| Leaf rust | Greenhouse | 385 | 385 |
| Powdery mildew | Greenhouse | 385 | 385 |
| Tan spot | Greenhouse | 280 | - |
| Stripe Rust | Field | - | 240 |
| Wheat soil-borne mosaic/wheat spindle stream mosaic | Field | - | 950 |
| Barley yellow dwarf | Field | - | 240 |
Understanding the Genetic Factors Underlying Rust Resistance in OSU Hard Red Winter Wheat
We created a germplasm collection of 619 breeding lines and cultivars from the OSU wheat breeding program. This collection was evaluated at the seedling stage in the greenhouse against United States leaf rust and stripe rust pathogen races and the adult plant stage in leaf rust and stripe rust nurseries in Oklahoma, Kansas and Washington. We observed higher frequencies of lines with leaf rust resistance at the adult plant stage than at the seedling stage. This suggests the presence of adult plant resistance (APR) in OSU breeding lines. Stripe rust resistance was due to the presence of APR genes. In the fall of 2024, this germplasm was planted in rust nurseries in Oklahoma (Stillwater and Lahoma) and Washington (Pullman and Central Ferry) for leaf rust/stripe rust evaluations in the spring of 2025.
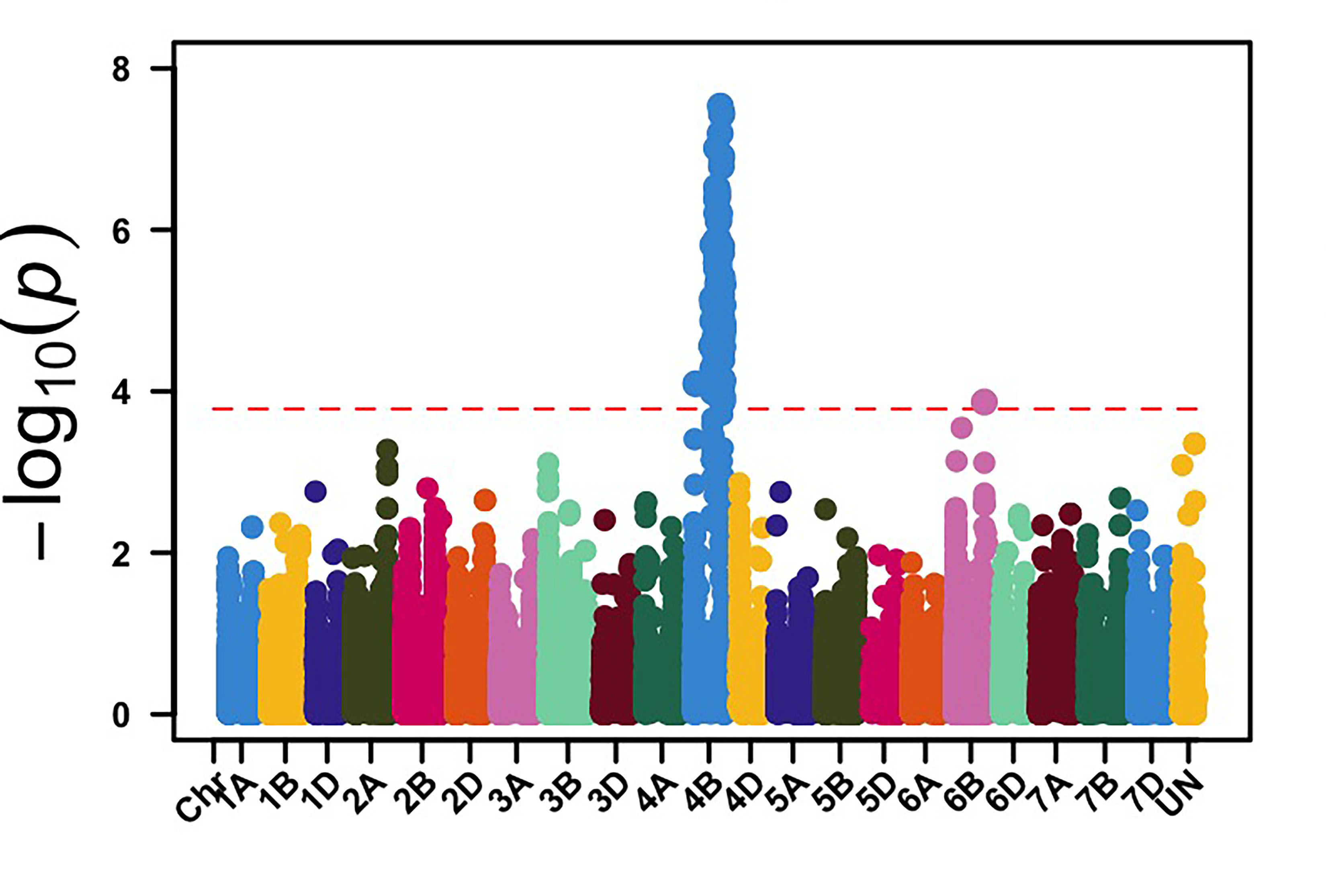
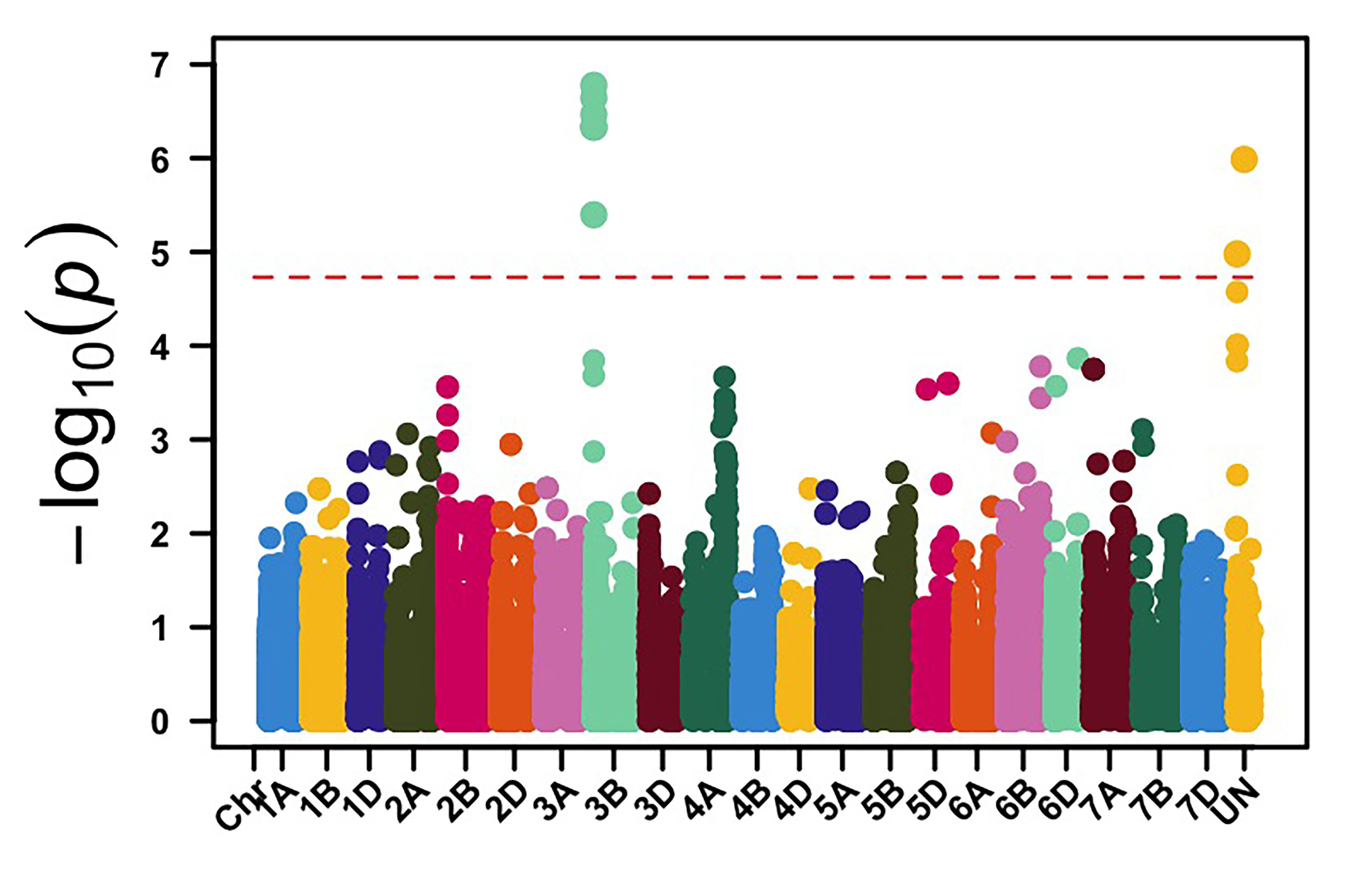
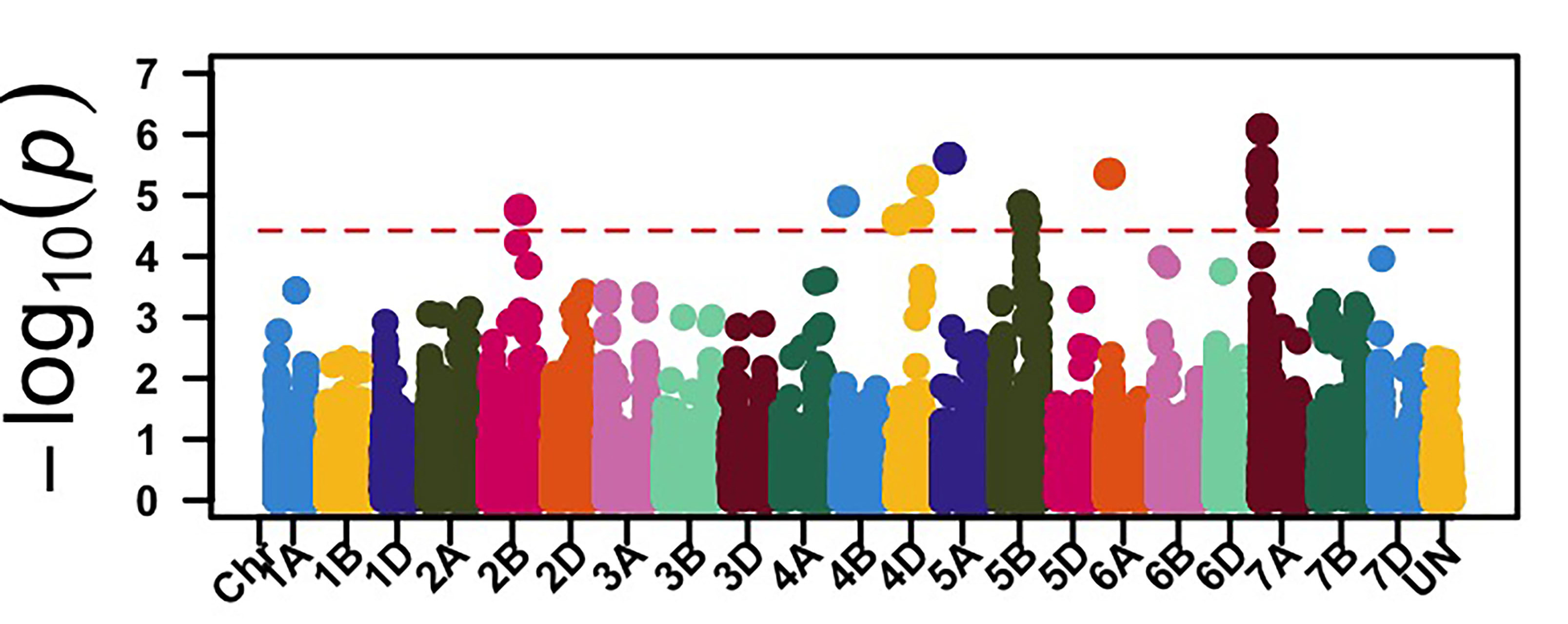
We genotyped this germplasm using genotyping by sequencing (GBS), and 39,286 molecular markers were retained for genome-wide association mapping (GWAS) to identify genomic regions associated with leaf rust and stripe rust resistance in OSU hard red winter wheat. GWAS, using molecular markers and stripe rust data collected in 2024, identified 113 genomic regions associated with stripe rust response. Among these loci, genomic regions on chromosome arms 3BS and 4BL showed the most significant associations with stripe rust response in multiple field environments (Figure 1). The APR stripe rust resistance (Yr) genes, known to be deployed in hard red winter wheat, are mainly Lr34/Yr18 and Lr46/Yr29, which are located on the chromosome arms 7DS and 1BL, respectively. Yr17 is a gene present in about 50% of this OSU wheat disease panel, however, virulence to Yr17 is present in the current stripe rust pathogen population. Our results showed that other effective Yr genes are likely present in OSU hard red winter wheat that have yet to be characterized.
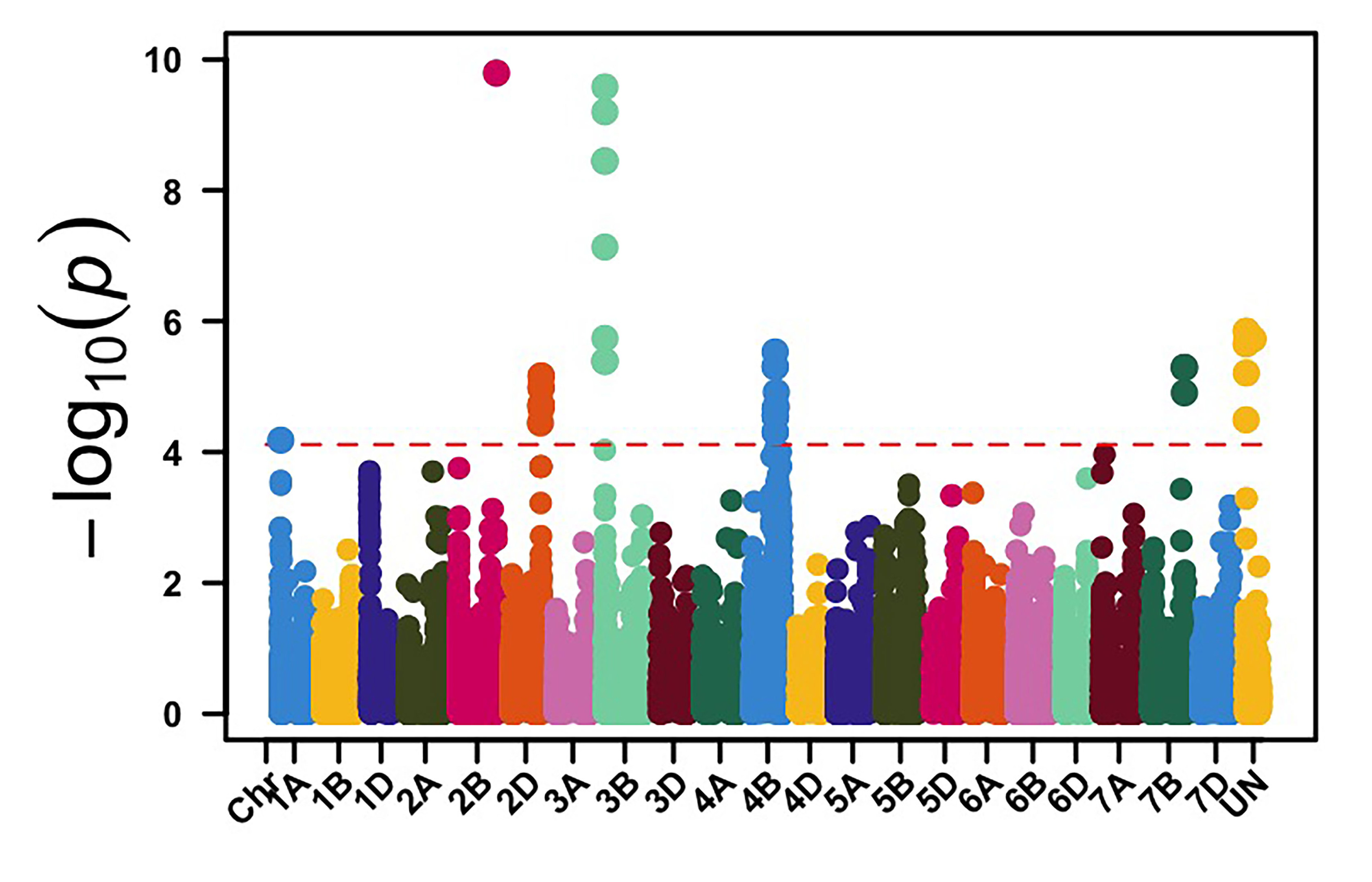
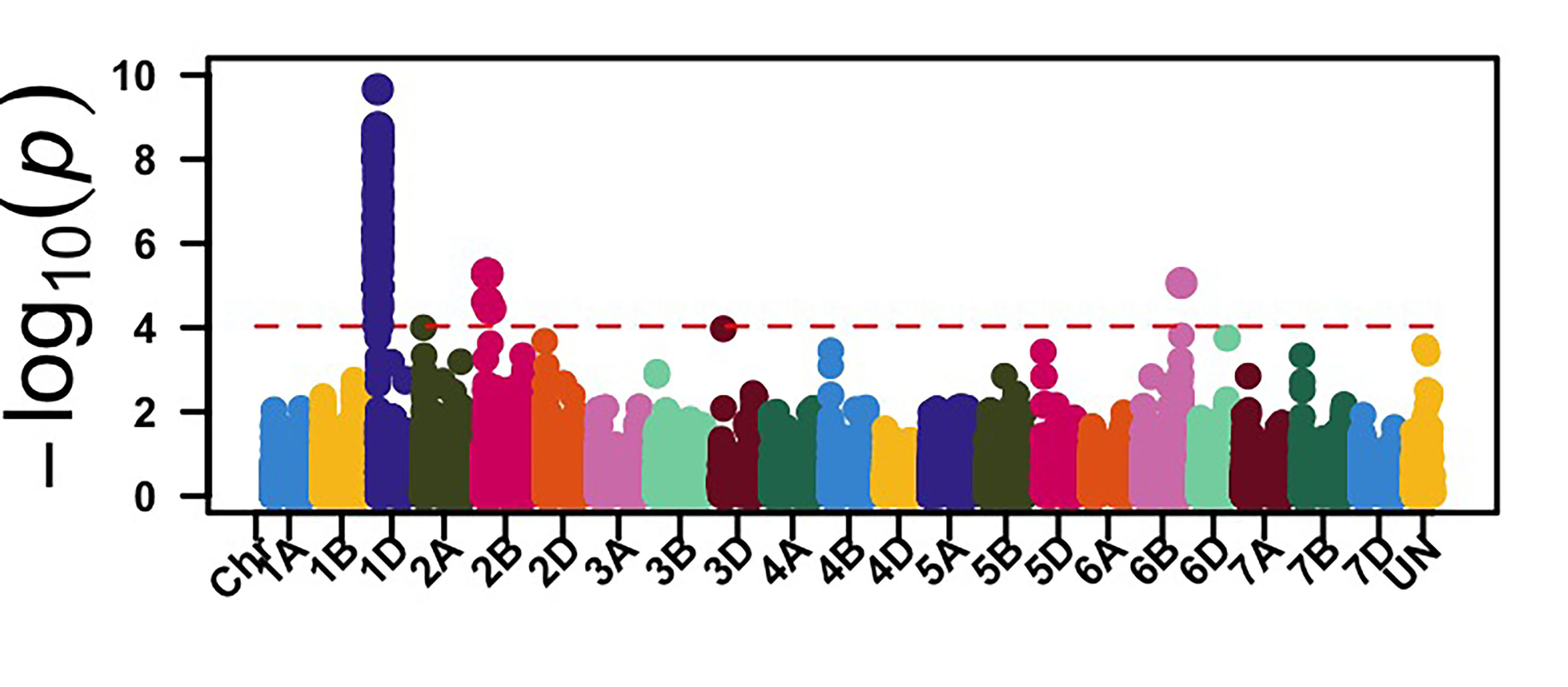
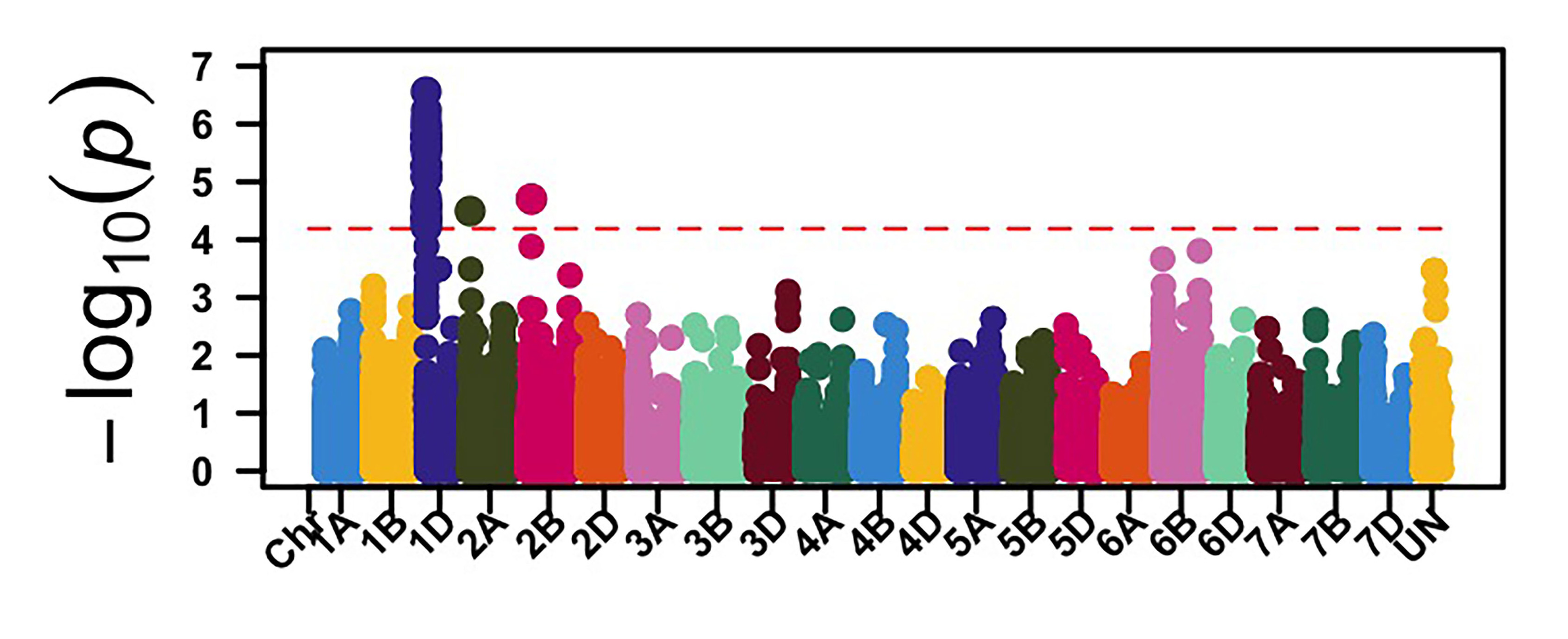
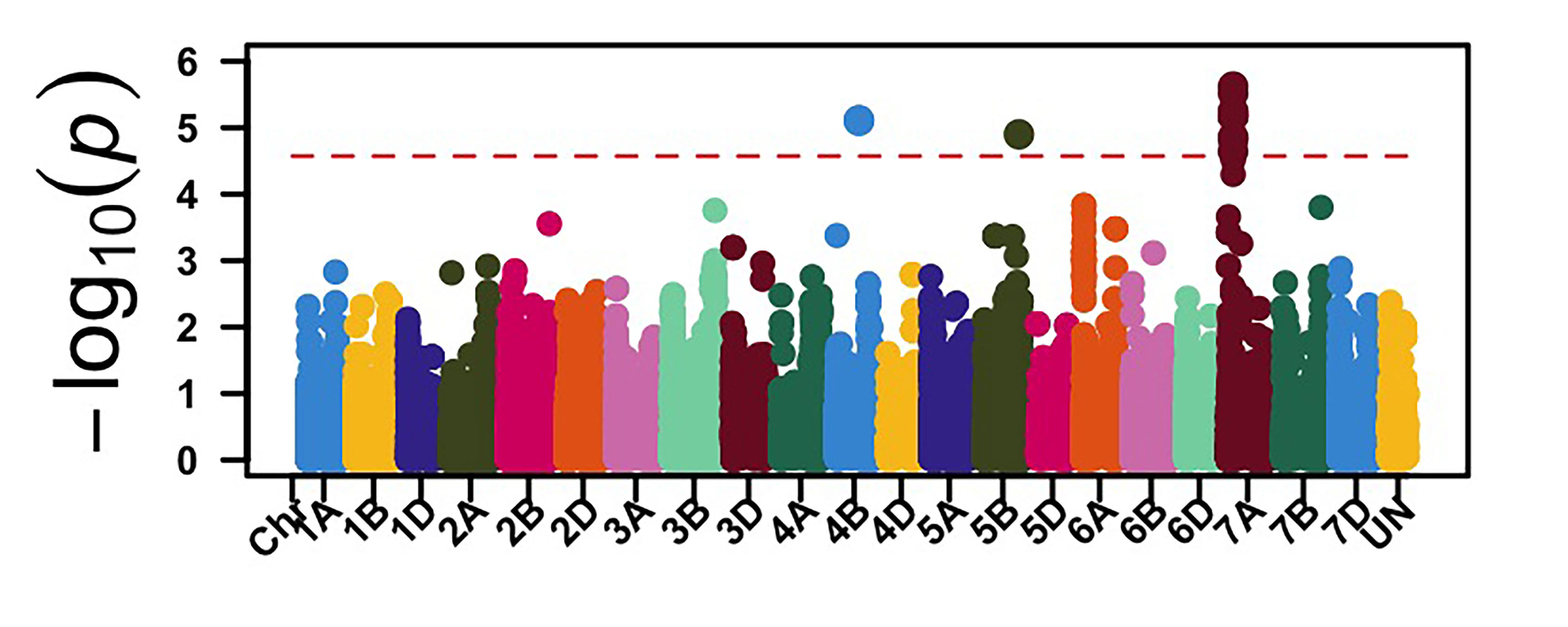
The GWAS identified 26 genomic regions associated with leaf rust resistance with two loci on chromosomes 1D and 2B associated with seedling leaf rust response against races MNPSD and MPPSD. Thus, a locus on chromosome 6B was associated with leaf rust resistance against race TNBJS. Genomic regions on chromosome arms 5BL and 7AS were associated with leaf rust response at the adult plant stage in Stillwater in 2023 and 2024 (Figure 2). The genomic region on chromosome arm 7AS had the most significant effect on leaf rust response in both field seasons in Stillwater. Further work is needed to characterize the leaf rust resistance gene on chromosome 7AS. Developing molecular markers associated with these identified leaf rust and stripe rust resistance loci is ongoing for potential use in marker-assisted selection.
Figure 1a-e. Genome-wide association mapping identified genomic regions on chromosome arms 3BS and 4BL associated with stripe rust response in multiple field environments in Oklahoma, Kansas and Washington.
Figure 1a. Pullman, WA
Figure 1b. Rossville, KS
Figure 1c. Chickasha, OK
Figure 1d. Central Ferry, WA
Figure 1e. Hutchinson, KS
Figure 2a-e. Genome-wide association mapping identified genomic regions associated with leaf rust response at the seedling stage against races MNPSD, MPPSD and TNBJS and the adult plant stage in the Stillwater field in 2023 and 2024.
Figure 2a. MNPSD
Figure 2b. MPPSD
Figure 2c. TNBJS
Figure 2d. Stillwater 2023
Figure 2e. Stillwater 2024
Understanding the Genetic Factors Underlying Septoria Nodorum Blotch Susceptibility in OSU Hard Red Winter Wheat
Septoria nodorum blotch (SNB) caused by Parastagonospora nodorum is a recurring disease that affects wheat in several regions worldwide. SNB is an emerging problem in Oklahoma, and very little is known about SNB resistance/sensitivity genes in hard red winter wheat in the southern Great Plains. P. nodorum is a necrotrophic fungus, which interacts with wheat in an inverse gene-for-gene manner. The recognition of P. nodorum necrotrophic effectors (NEs) by wheat sensitivity genes leads to host susceptibility. To date, five sensitivity genes (Tsn1, Snn1, Snn3-B1, Snn3-D1 and Snn5) and five effector genes (SnToxA, SnTox1, SnTox3, SnTox5 and SnTox267) have been cloned. Our objectives were to identify the reactions of OSU breeding lines and cultivars against SNB and to identify genomic regions associated with SNB response using GWAS.
The OSU panel of 619 wheat experimental lines and cultivars was evaluated at the seedling stage for their reactions against two P. nodorum isolates and three P. nodorum effectors (ToxA, Tox1 and Tox3). In addition, this germplasm was screened using diagnostic markers for the susceptibility genes Tsn1 and Snn1 (Figure 3). The results showed that 55% and 73% of the OSU panel do not carry Tsn1 and Snn1. OSU cultivars like Big Country, Uncharted and Green Hammer do not carry the sensitivity gene Tsn1, whereas multiple OSU cultivars do not carry Snn1 (Table 2). Genotypes that are sensitive to ToxA must carry the sensitivity gene Tsn1. In our study, the accuracy of Tsn1 markers fcp991 and fcp992 in predicting sensitivity to ToxA was perfect (98%-99%). Snn1 interacts with Tox1 to cause sensitivity. Although the Snn1 molecular marker can perfectly predict the presence/absence of Snn1, there are insensitive forms of Snn1 like those present in Jagger, OK Bullet, Smith’s Gold and High Cotton (Table 2). Insensitive Snn1 forms will likely increase in frequencies in future breeding cycles due to the prevalence of Smith’s Gold and High Cotton in up-and-coming experimental lines.
| Cultivar Name | Toxin infiltration assay - ToxA | Toxin infiltration assay - Tox1 | Toxin infiltration assay - Tox3 | Molecular marker assay - Tsn1 markers - fcp991 | Molecular marker assay - Tsn1 markers - fcp992 | Molecular marker assay - Snn1 marker |
|---|---|---|---|---|---|---|
| Duster | S | I | I | Tsn1+ | Tsn1+ | Snn1- |
| Bentley | I | I | I | Tsn1- | Tsn1- | Snn1- |
| Big Country | I | I | I | Tsn1- | Tsn1- | Snn1- |
| Breakthrough | S | S | - | Tsn1+ | Tsn1+ | Snn1+ |
| Butler's Gold | S | I | S | Tsn1+ | Tsn1+ | Snn1- |
| DoubleStop CL+ | S | I | I | Tsn1+ | Tsn1+ | Snn1- |
| Duster | S | I | S | Tsn1+ | Tsn1+ | Snn1- |
| Gallagher | S | I | I | Tsn1+ | Tsn1+ | Snn1- |
| Green Hammer | I | S | I | Tsn1- | Tsn1- | Snn1+ |
| Iba | S | I | S | Tsn1+ | Tsn1+ | Snn1- |
| Jagalene | S | I | S | Tsn1+ | Tsn1+ | Snn1- |
| Jagger | S | I | I | Tsn1+ | Tsn1+ | Snn1+ |
| OK Bullet | S | I | - | Tsn1+ | Tsn1+ | Snn1+ |
| OK Corral | I | I | S | Tsn1- | Tsn1- | Snn1- |
| Overley | I | I | S | Tsn1- | Tsn1- | Snn1- |
| Showdown | S | I | I | Tsn1+ | Tsn1+ | Snn1- |
| Smith's Gold | S | I | S | Tsn1+ | Tsn1+ | Snn1+ |
| Uncharted | I | I | S | Tsn1- | Tsn1- | Snn1- |
| High Cotton | S | I | I | Tsn1+ | Tsn1+ | Snn1+ |
| Orange Blossom CL+ | I | I | S | Tsn1- | Tsn1- | Snn1- |
| Paradox | S | S | I | Tsn1+ | Tsn1+ | Snn1+ |
| Firebox | S | I | I | Tsn1+ | Tsn1+ | Snn1- |
S: Sensitive (susceptible); I= insensitive (resistant)
Tsn1+: The sensitivity gene Tsn1 is present; Tsn1-: The sensitivity gene Tsn1 is absent.
Snn1+: The sensitivity gene Snn1 is present; Snn1-: The sensitivity gene Snn1 is absent.
Tsn1 and Snn1 markers were developed by Justin Faris’ lab at the USDA-Agricultural Research Service in Fargo, North Dakota.
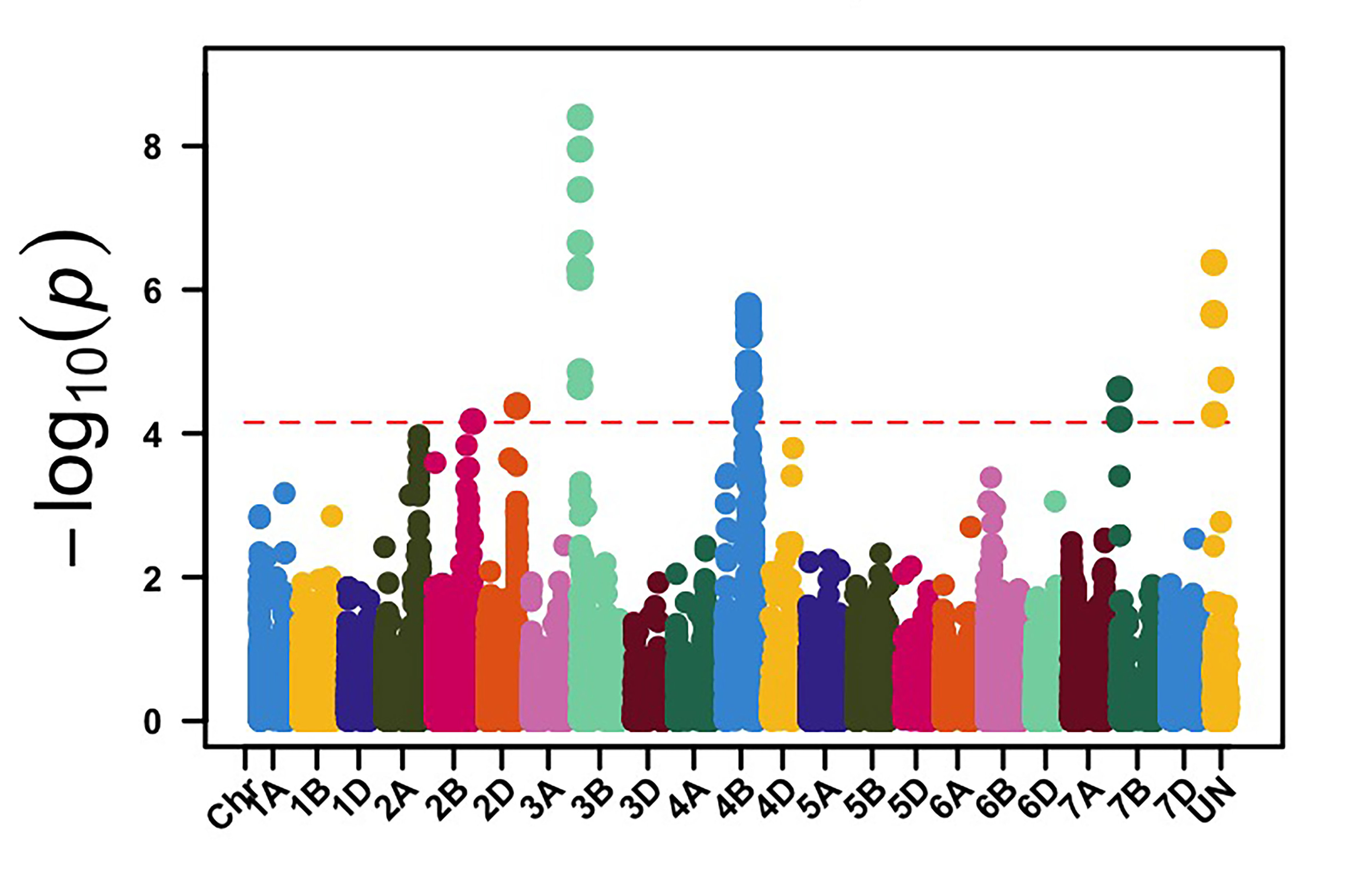
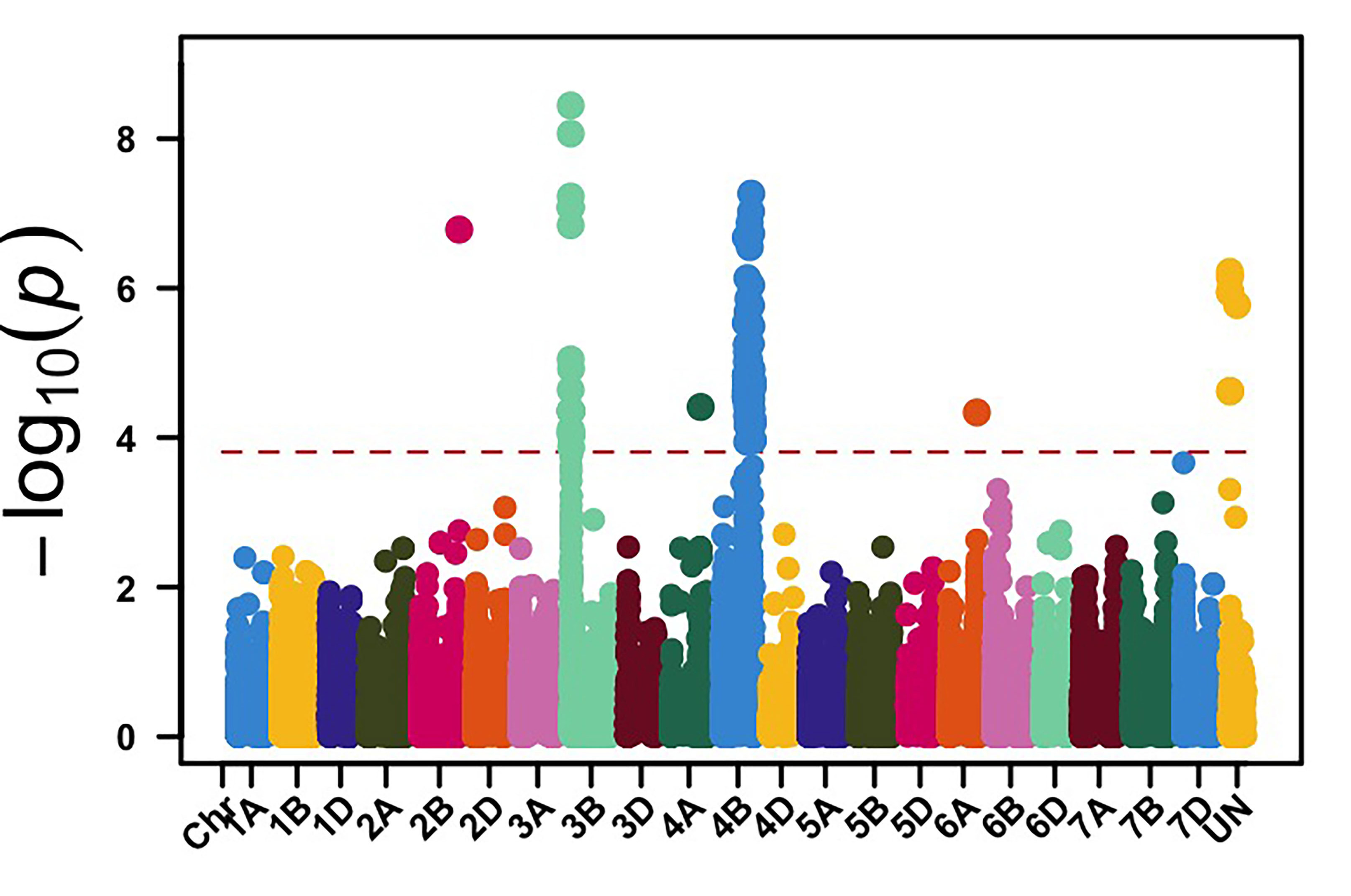
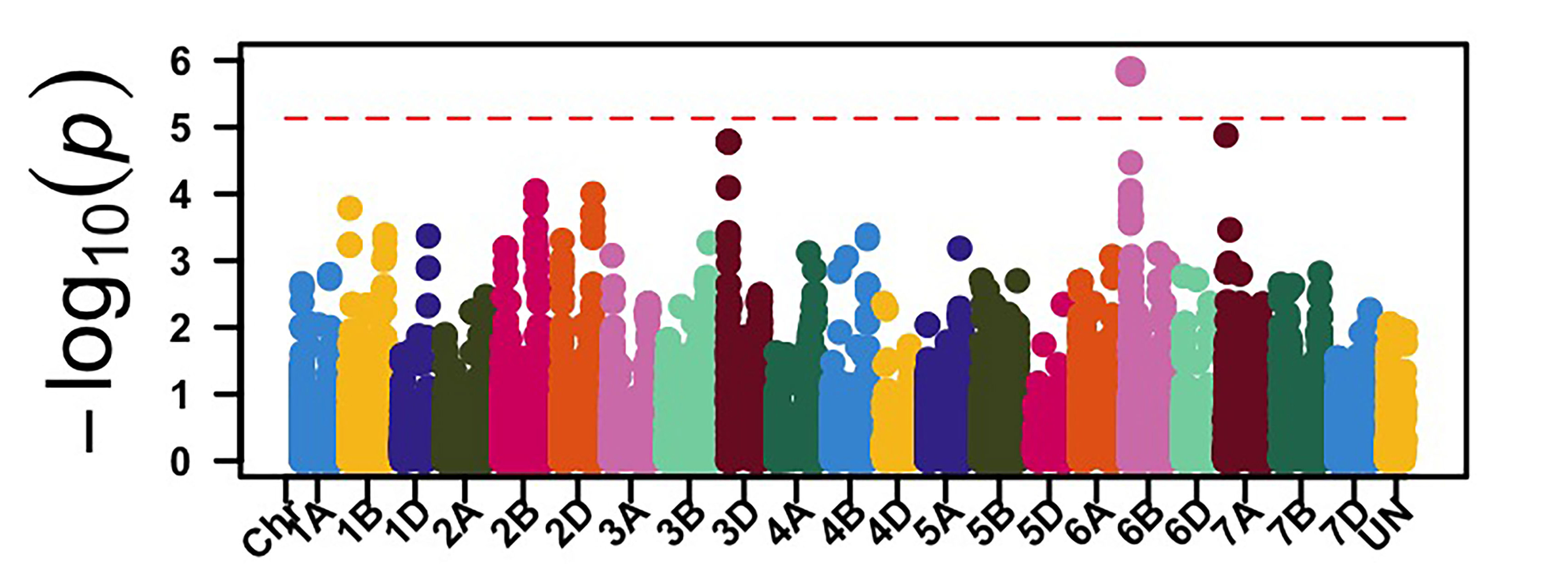
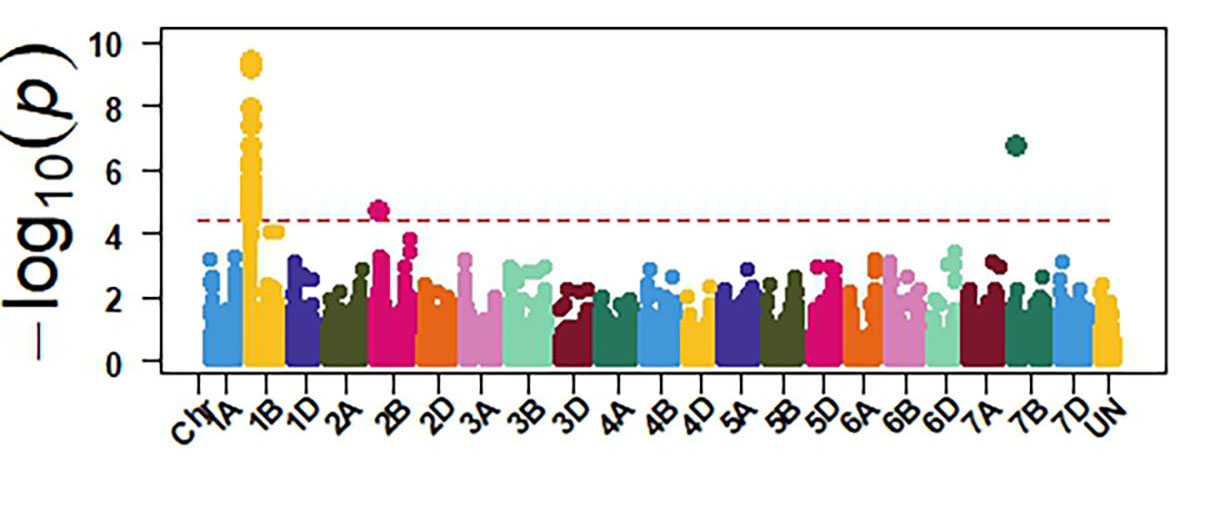
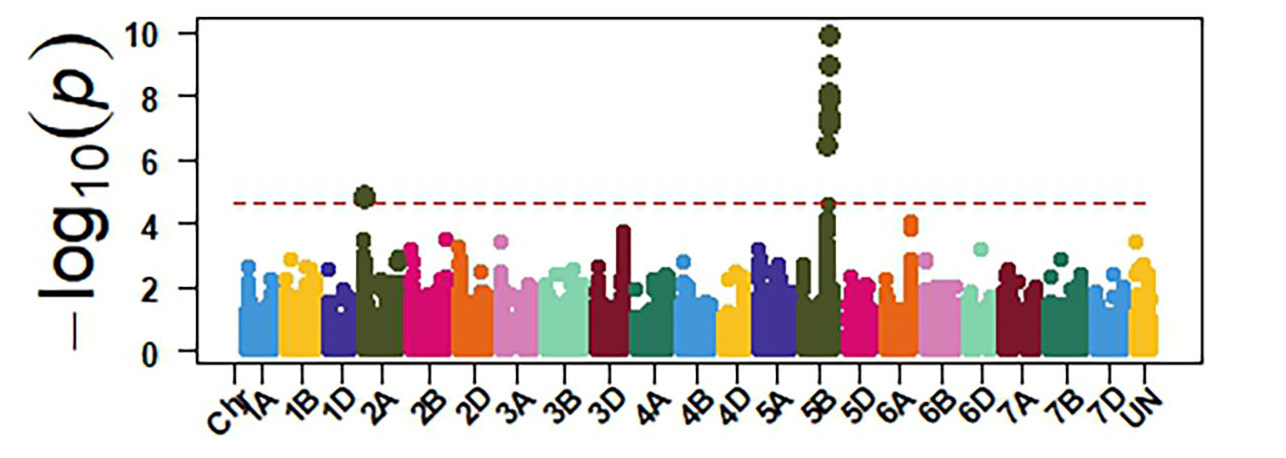
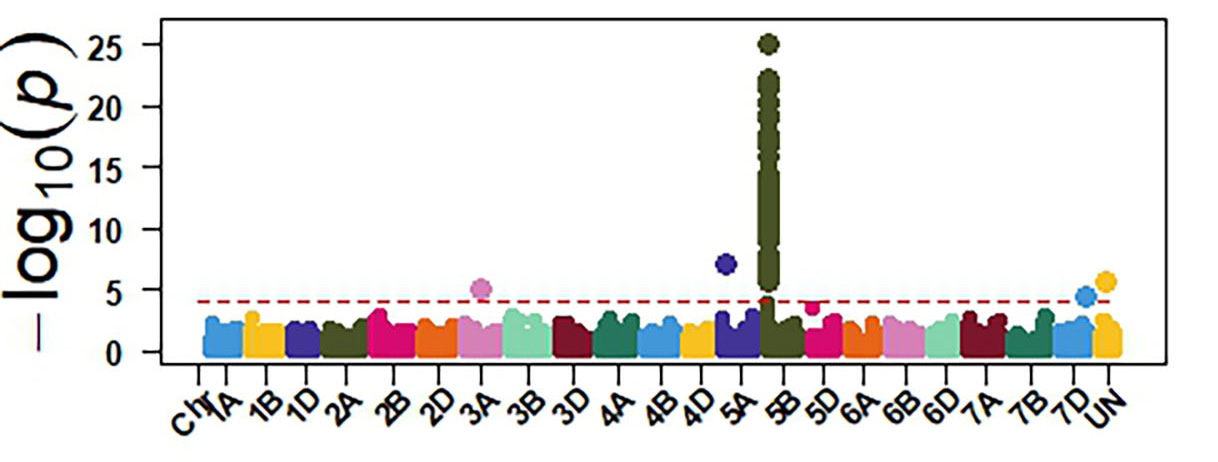
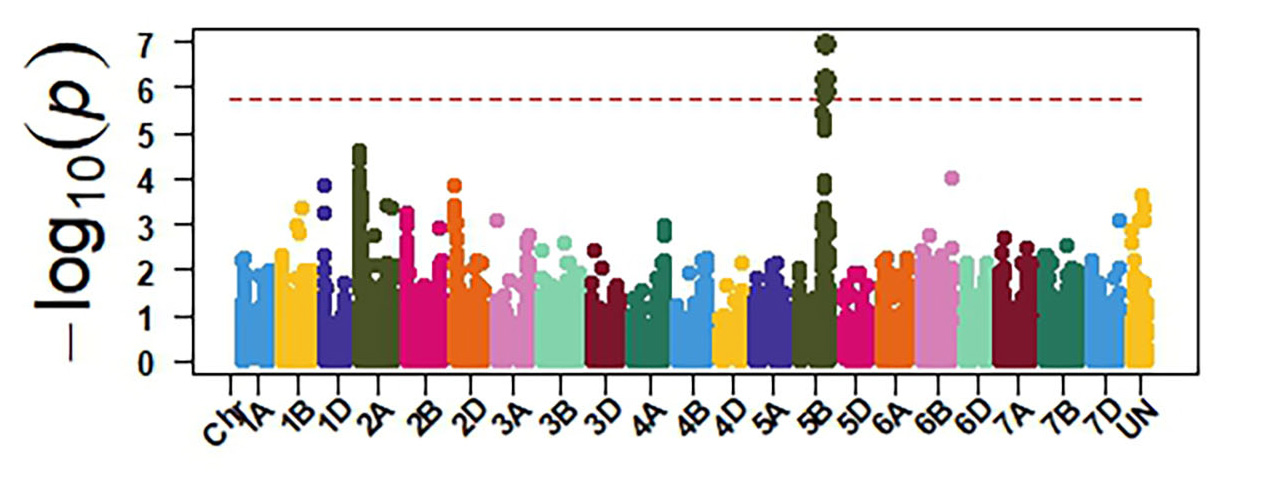
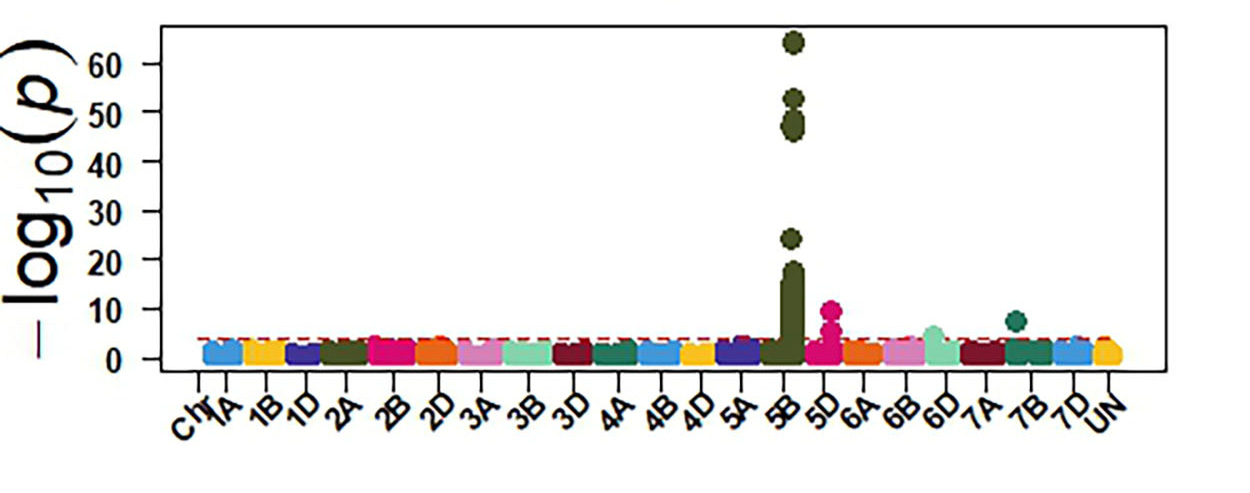
Molecular markers generated from genotyping by sequencing and the phenotypic data were used for GWAS to identify genomic regions associated with response to SNB. The GWAS confirmed the presence of three sensitivity genes Tsn1, Snn1 and Snn3 in OSU wheat. Tsn1 has the largest effect in explaining sensitivity to the two P. nodorum isolates (Figure 4). Therefore, eliminating Tsn1 from OSU wheat using molecular markers will enhance resistance to SNB. Tsn1 causes susceptibility to two other fungal diseases, tan spot and spot blotch. Therefore, eliminating Tsn1 from the OSU breeding program will enhance resistance against these three diseases. Molecular markers identified from our GWAS can facilitate the development of a diagnostic molecular marker for Snn3, which is not currently available.
Figure 4a-e. GWAS identified sensitivity genes to Septoria nodorum blotch in OSU hard red winter wheat. The OSU panel was evaluated at the seedling stage for their reactions against two P. nodorum isolates OKG16 Sn1 and OKG16 Sn13 and three effectors ToxA, Tox1 and Tox3 that interact with Tsn1 (peak on chromosome arm 5BL), Snn1 (peak on chromosome arm 1BS) and Snn3 (peak on chromosome arm 5BS) to cause susceptibility.
Figure 4a. Tox1
Figure 4b. OKG16 Sn1
Figure 4c. Tox3
Figure 4d. OKG16 Sn13
Figure 4e. ToxA